Certification Requirements
NeoMed features the export of patient electronic health data in a standard computable and human-readable Consolidated Clinical Document Architecture (C-CDA) standard format. This functionality fulfills export summary capability under 2015 Edition CURES Update Certification measure (b)(10).
Single Patient
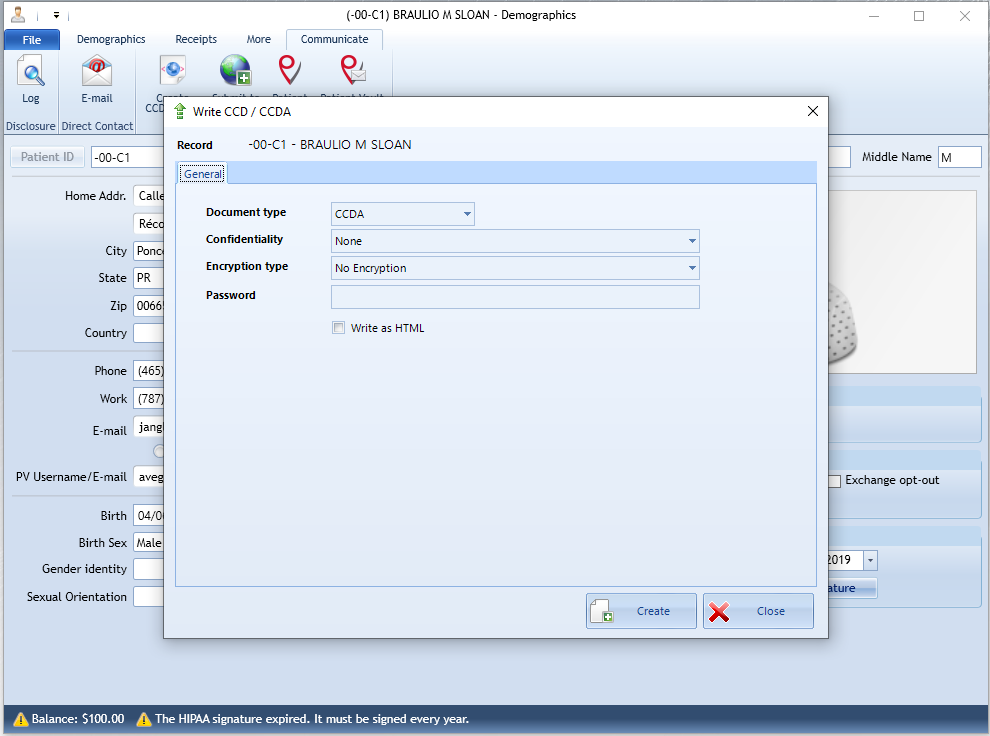
NeoMed allows administrators and designated users in a facility to export electronic health information (EHI) for a single patient. On demand reports based user-submitted queries using the reports module.
NeoMed 4 | NeoMed 3 |
|---|---|
|
|
NeoMed 4 | NeoMed 3 |
|---|---|
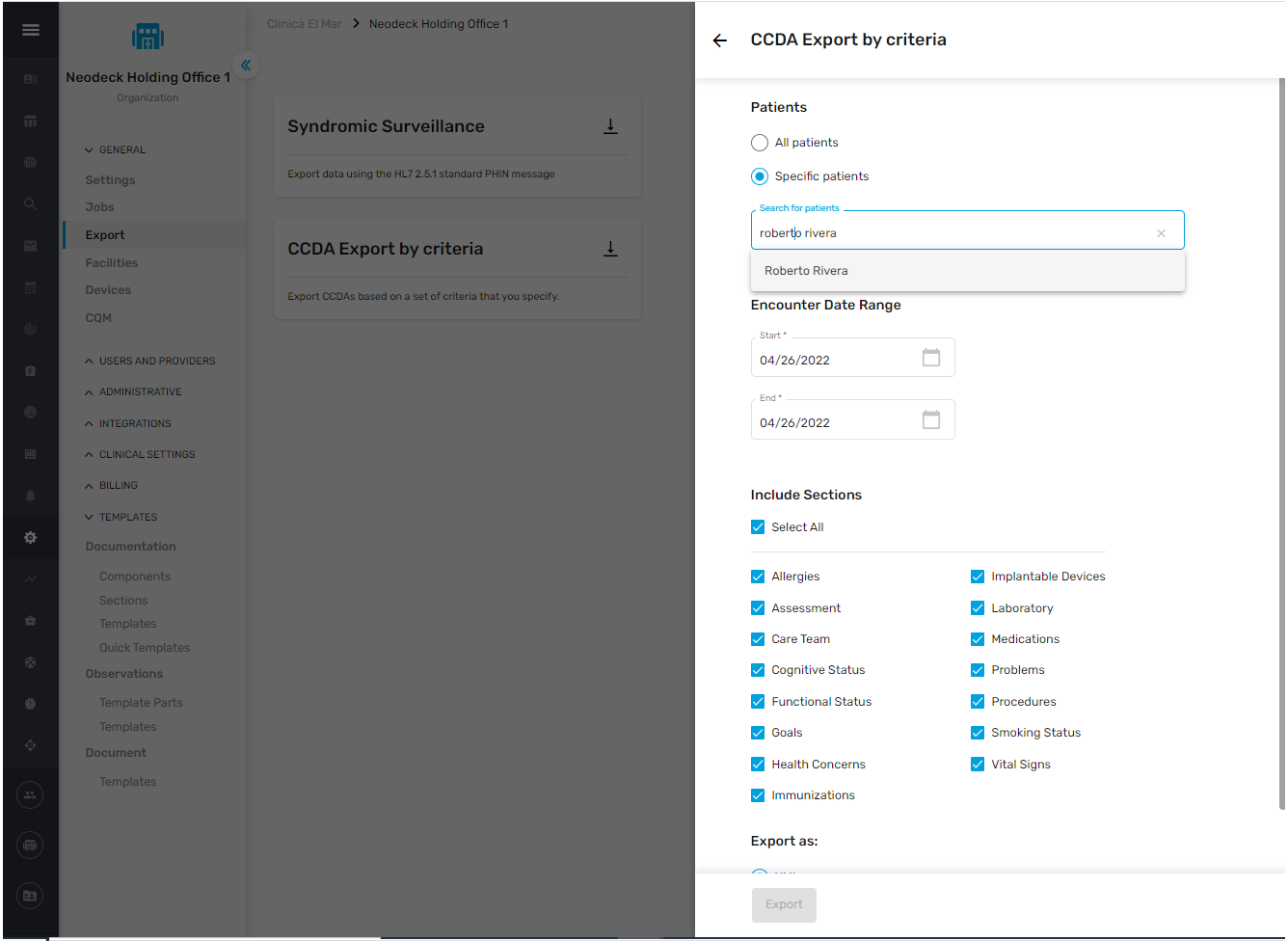
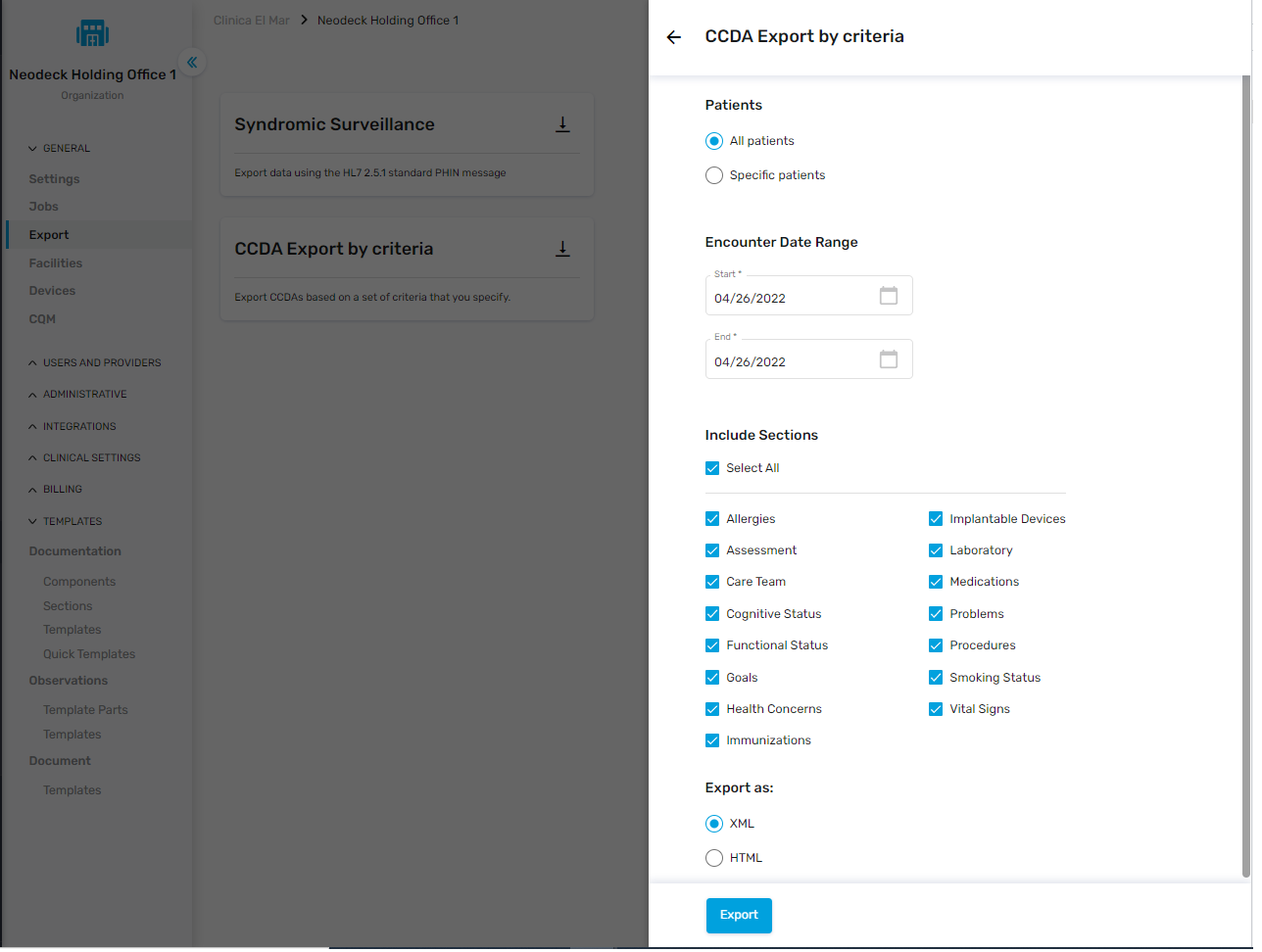
Multiple Patients
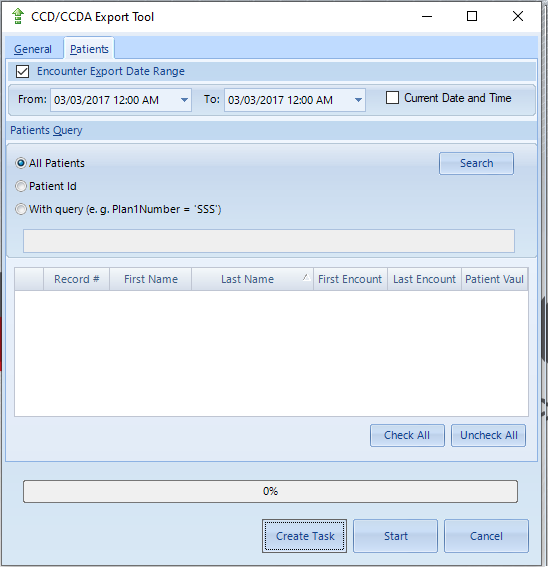
NeoMed users can also export electronic health information (EHI) for multiple patients.
NeoMed 4 | NeoMed 3 |
|---|---|
Computable and Human-readable Format
The electronic file is generated in CCDA version 1.0 Extensible Markup Language (XML) format that is both a computable and a human-readable format. NeoMed 4 also offers EHI exported in Hypertext Markup Language (HTML) or web page format.
FHIR Implementation
Bulk
NeoMed FHIR server creates a single-patient FHIR resource DocumentReference and supports FHIR Bulk Data EHI Export for patient population as described in § 170.315(b)(10)(ii).
CCDA XML version 2.1
NeoMed FHIR server supports bulk data export downloads in C-CDA 2.1 XML format.
USCDI version 1.0
Includes new US Core Data for Interoperability Version 1 (USCDIv1) data elements:
Demographics, Pediatric Vital Signs, Provenance, Pediatrics, and Clinical Notes
The C-CDA Clinical Notes section can contain a Consultation Note, Discharge Summary Note, History & Physical, Imaging Narrative, Laboratory Report Narrative, Pathology Report Narrative, Procedure Note, or a Progress Note.